Research Projects
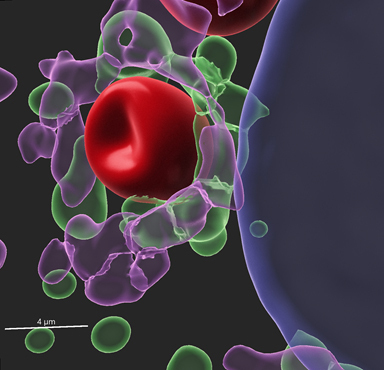
Characterization of the intracellular niche of Cryptococcus
When cells internalize microbes, they end up in a compartment called the phagosome. This phagosome undergoes a series of maturation processes that ends up with fusion with a lysosome, resulting in destruction of the microbe. However, although C. neoformans is internalized into a phagosome, it can survive and replicate. The precise identity of the cryptococcal-containing phagosome is unclear, and studies of this niche may explain how this fungus avoids destruction, and how to promote it. We hypothesize that Cryptococcus targets the Rab GTPases and phosphoinositides responsible to regulate the endolysosomal pathway, and we are actively studying this in various disease-relevant cell types.
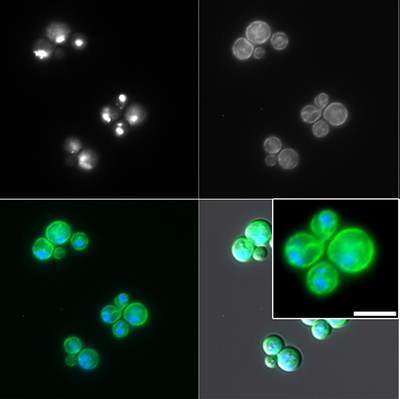
Characterization of novel virulence factors in Cryptococcus
We have identified uncharacterized fungal genes that are involved in important pathogenic processes. We are actively studying these genes to determine their biological function and their role in disease. Two of these genes are involved in palmitoylation and in antifungal resistance, both of which are important processes in the fungal pathogenesis field but also can provide insights into more fundamental biology applicable to other pathogens. We also are starting to work with the cryptococcal vacuole as we found that in the pfa4 mutant (Santiago-Tirado et al., 2015) it is fragmented and hence may contribute to the virulence defects exhibited by this mutant.
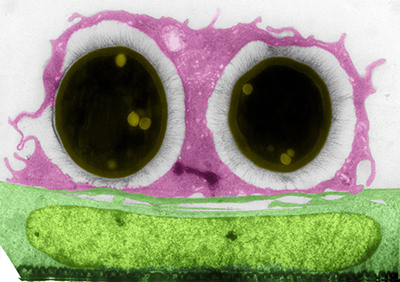
Understanding host-fungal interactions at different cellular barriers
C. neoformans is acquired by inhalation, however the fatal pathology occurs at the brain, hence the fungus must cross several cellular barriers as it goes from the lungs to the brain. We have found that one common way it can do this, at least at the blood-brain barrier (BBB), is by using host monocytes as Trojan horses. In our newest project, we have obtained a recent air-liquid interface (ALI) model of the lung to study the interactions of the fungus with the epithelial cells of the alveoli. We plan to compare/contrast the interactions at these two sites to start understanding how Cryptococcus is able to cross cellular barriers and disseminate.
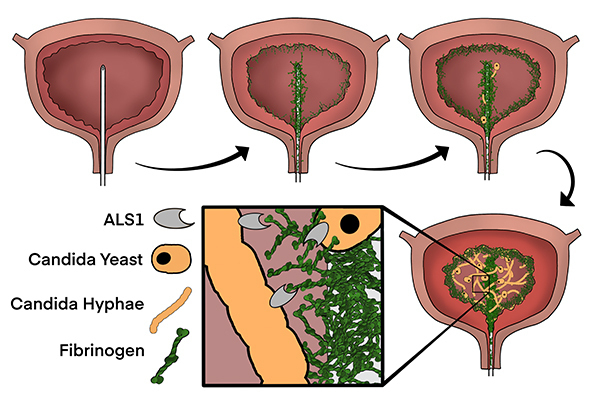
Understanding the fungal and host factors that promote fungal CAUTI
Urinary catheterization is one of the most common healthcare-related procedures. Interestingly, few pathogens can cause urinary disease without a catheter in place. Candida albicans is one of these pathogens that, in recent years, has become an increasingly common catheter-associated urinary tract infection (CAUTI) pathogen, yet most studies have focused on bacterial uropathogens. To start understanding the pathogenesis of Candida CAUTI, we joined forces with the lab of Dr. Flores-Mireles to, for the first time, investigate the fungal and host factors playing a role specifically in the catheterized bladder. We hope to uncover novel areas for intervention and prevention of fungal CAUTI, and even of other fungal infections involving a medical device such as venous catheters.